PK/PD Analysis Services
PK/PD Analysis Services
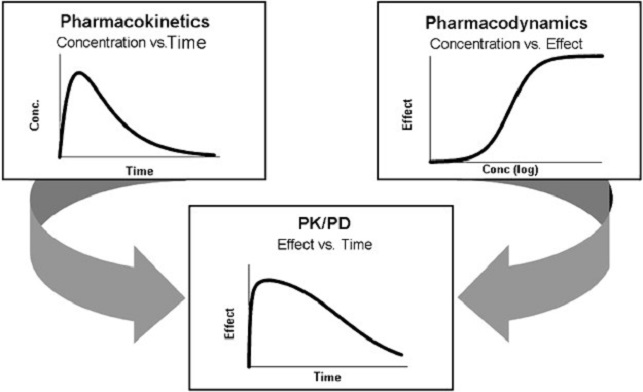
Clinical pharmacology is the study of how various drugs interact with a real patient’s body. Pharmacokinetics and pharmacodynamics are two of the most generic subfields in the area of clinical pharmacology. Pharmacokinetics and pharmacodynamics, which are both crucial in establishing the safety and efficacy of a drug, quantify and describe the complex biochemical interactions that occur between the body’s natural processes and the chemical composition of a pharmaceutical drug.
Regulatory agencies like the Food and Drug Administration are responsible for both the approval of new drugs and the decision of whether or not an existing drug should be taken off the market (FDA). Regulatory agencies must also make sure that all drugs currently on the market are both efficient and secure for human use. Any examination into the effectiveness and safety of any pharmaceutical product must include a consideration of pharmacokinetics and pharmacodynamics.
Examples of our PK and PD services include the following:
- PK/PD investigation and report of findings
- Pre-clinical ADME and clinical study design and analysis
- Simulated doses
- Risk assessment
- creating PK reports that are either standalone PK reports or integrated inside PK studies
- Production of CDISC datasets and data management
Reporting Analyses that Do Not Take Into Account Compartments and Analysis of PK/PD Interactions Information on pharmacokinetics (PK) is frequently gathered from investigations of this kind (i.e., the rate and extent of absorption and elimination).
Any and all clinical data provided to relevant health authorities must follow the format set by the Clinical Data Interchange Standards Consortium (CDISC). This kind of reporting falls under the purview of our data managers, who are also extremely proficient in CDISC translation.
Speak with our PK/PD Experts for Advice
Cloud Data Vision is the leading PK/PD science consulting company in terms of our expertise and commitment to provide our customers outstanding service. To advance efforts in drug research, we provide superior clinical & nonclinical noncompartmental PK analysis. We offer a variety of reporting options, from simple outputs to thorough pharmacokinetic reports that are ready for submission.
To learn more about the many ways that our thorough PK analyses could benefit your programme, get in touch with us right away. We enjoy working closely with our clients to determine the solutions that are both the most suitable and the most affordable for each unique drug development programme.
Clinical Data Services
IT SERVICES

