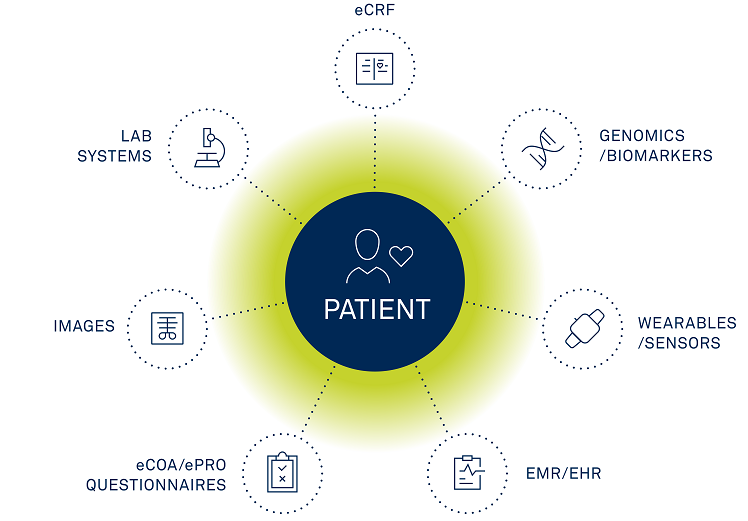
Clinical data management is the process of gathering and maintaining research data in accordance with regulatory standards to obtain accurate, comprehensive, and error-free information (sometimes abbreviated as CDM). The goal is to gather as much of this kind of data for analysis as is permitted by law while abiding by all relevant local, state, and federal regulations.
Clinical data management, or CDM, is a field that has grown as a result of the expectations placed on it by both regulatory bodies and the pharmaceutical industry. Regulatory authorities have reacted to the ongoing desire to “fast-track” the development of pharmaceutical goods by requiring that quality-assurance standards be followed while obtaining the data that will be used in the process of evaluating possible new treatments.
Examples of such standards that are crucial to the Clinical Data Model include the Clinical Data Acquisition Standards Harmonization and the Study Data Tabulation Model Implementation Guide for Human Clinical Trials (SDTMIG) (CDM). The Clinical Data Interchange Standards Consortium (CDISC) created both of these standards (CDASH). In the United States, the former is currently required by law. The Food and Drug Administration is known as FDA (FDA). The latter created a uniform format for data collection throughout research, making it simpler to organise and evaluate data submission.
Specialized software solutions are used in clinical data management (CDM) to create audit trails, reducing disparities even in the largest and most challenging clinical investigations.
